Have you ever wondered why oil and water don’t mix? Chemistry provides us with the answer to this question, and it is quite an interesting one. Oil and water are two substances with different chemical properties that makes it impossible for them to combine. In this article, we will explore the chemistry behind why oil and water don’t mix and why this phenomenon is so important to our everyday lives.
When it comes to chemistry, the answer to why oil and water don’t mix is all about polarity. Polar molecules are attracted to one another, while non-polar molecules are not. Oil is a non-polar molecule, meaning that it has no positive or negative charge. Water, on the other hand, is a polar molecule, meaning it has a slight positive charge at one end and a slight negative charge at the other. This means that water molecules are attracted to one another, while oil molecules are not. This is why oil and water cannot mix – because they have different properties that prevent them from combining.
Oil and water don’t mix due to their different chemical properties. Oil molecules are non-polar, meaning they repel each other and will not mix with water molecules, which are polar. Because of this, oil and water form two separate layers when mixed together.
Why Don’t Oil and Water Mix Chemistry?
Oil and water are two substances that are widely used in everyday life. They are two of the most important components of life and are used in many different ways. However, they are two substances that do not mix and remain separate no matter how much effort is put into trying to combine them. This is due to their unique chemical makeup that causes them to repel each other. Understanding the chemistry behind why oil and water don’t mix can help to explain why this phenomenon occurs.
The Different Chemical Makeup of Oil and Water
Oil and water have different chemical properties, which is why they do not mix. Oil is hydrophobic, meaning it does not attract water molecules. This is because the oil molecules are non-polar, meaning the molecules have an even distribution of electrons. Water molecules, on the other hand, are polar, meaning that the electrons in the molecules are unevenly distributed. The unevenness of the molecules causes them to be attracted to each other, forming a bond. Because oil molecules are non-polar, they are not attracted to the water molecules and remain apart.
The different chemical makeup of oil and water is the main reason why they do not mix. The unique properties of each substance cause the molecules to be repelled by one another, making it impossible for them to combine and form a solution. This is why oil and water remain separate, no matter how much effort is put into trying to mix them.
Surface Tension and the Interfacial Layer
Surface tension is another phenomenon that plays a role in why oil and water do not mix. Surface tension is the force that causes molecules of a liquid to stick together and form a layer on the surface of the liquid. Water has a higher surface tension than oil, which is why the molecules of oil are pushed away from the water molecules by the surface tension of the water. This creates an interfacial layer between the two liquids, which prevents them from mixing.
The interfacial layer also helps to explain why oil and water are able to form emulsions. An emulsion is a mixture of two liquids that are held together by the interfacial layer. This layer helps to keep the two liquids from separating, which is why oil and water can form emulsions but cannot form a solution.
The Hydrophilic-Lipophilic Balance
The hydrophilic-lipophilic balance (HLB) is another factor that affects why oil and water do not mix. The HLB is the measure of the relative hydrophobicity and hydrophilicity of a molecule. Molecules with a higher HLB are more hydrophilic, while molecules with a lower HLB are more hydrophobic. Oil molecules have a very low HLB, while water molecules have a very high HLB, which is why the two substances do not mix.
The HLB also helps to explain why oil and water can form emulsions. Emulsions are formed when the molecules of one liquid are attracted to the molecules of another liquid with a different HLB. In the case of oil and water, the oil molecules are attracted to the water molecules, forming an emulsion. The emulsion is held together by the interfacial layer, which prevents the two liquids from separating.
Interactions between Oil and Water Molecules
The interactions between oil and water molecules are another factor that affects why the two substances do not mix. Water molecules have a strong attraction to each other and to other molecules, while oil molecules have a weak attraction to each other and to other molecules. This means that when oil and water molecules come into contact with each other, the water molecules will be attracted to each other and will remain together, while the oil molecules will be repelled and will remain apart.
This is why oil and water molecules will remain separate no matter how much effort is put into trying to mix them. The strong attraction of the water molecules and the weak attraction of the oil molecules cause the two substances to remain apart, preventing them from forming a solution.
Frequently Asked Questions About Why Don’t Oil and Water Mix Chemistry?
Below are some of the most frequently asked questions about why oil and water don’t mix:
What is the chemistry behind why oil and water don’t mix?
The chemistry behind why oil and water don’t mix is due to the different chemical properties of each substance. Oil is composed of molecules that are non-polar, meaning that the molecules are not attracted to water molecules. On the other hand, water molecules are polar, meaning that they are attracted to one another. This creates an environment where oil molecules are not attracted to the water molecules, and therefore do not mix.
Additionally, the oil molecules are bigger than the water molecules, which means that the oil molecules are not able to fit between the water molecules. This means that the oil molecules are unable to move through the water molecules, which prevents them from mixing.
What happens when oil and water are mixed?
When oil and water are mixed, the oil molecules will float on the surface of the water. This is because the oil molecules are less dense than the water molecules, and thus they are pushed to the surface. The oil molecules will form a layer on top of the water, which prevents them from mixing.
Additionally, the oil molecules will not dissolve in the water, as they are not attracted to the water molecules. This means that the oil molecules will remain separate from the water molecules, and will not mix.
Are there any instances where oil and water can mix?
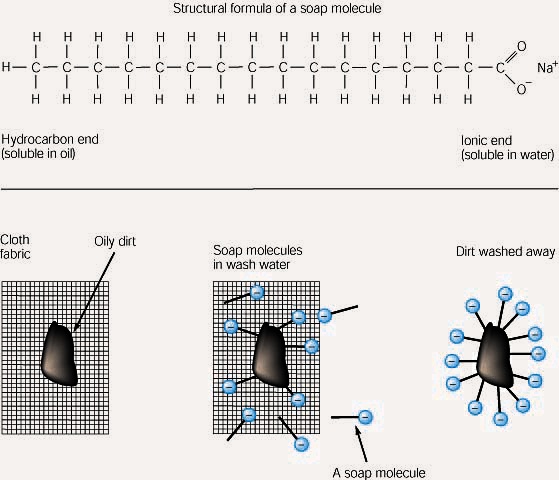
Yes, there are instances where oil and water can mix. This can be achieved through the use of surfactants, which are molecules that have both a polar and a non-polar part. The polar part is attracted to water molecules, while the non-polar part is attracted to oil molecules.
When a surfactant is added to oil and water, the surfactant molecules will form a bridge between the oil and water molecules. This bridge will allow the oil molecules to move through the water molecules, and thus allow the oil and water molecules to mix.
Why is it important to know why oil and water don’t mix?
It is important to know why oil and water don’t mix as it can help us to understand how to best use these substances. Knowing why oil and water don’t mix can help us to understand why it is important to use surfactants to mix oil and water, and can also help us to understand why oil and water should be kept separate.
Additionally, understanding why oil and water don’t mix can also help us to understand why oil and water can be used to clean surfaces, as the oil can be used to break down dirt and grime, while the water can be used to rinse it away.
Can oil and water be separated?
Yes, oil and water can be separated. This can be done through a process called decantation, which involves pouring the mixture into a container and allowing the oil to float to the top. Once the oil has floated to the top, it can be carefully removed and the water can be poured off.
Additionally, oil and water can also be separated through the use of filters. This involves pouring the mixture through a filter, which will trap the oil molecules, while allowing the water molecules to pass through. This allows the oil and water to be separated, without the need for manual decantation.

Why don’t oil and water mix? – John Pollard
In conclusion, the reason why oil and water don’t mix is because they have different properties. Oil is hydrophobic, meaning it is not soluble in water, while water is hydrophilic, meaning it is soluble in water. This is due to the chemical makeup of both substances, which is why the two remain separate when combined. Oil’s hydrophobic nature means that it is repelled by water molecules, while the water molecules are attracted to each other, forming a cohesive bond. This explains why, even when shaken or stirred together, oil and water will eventually separate.
The chemical incompatibility between oil and water plays an important role in everyday life. The ability to separate oil from water makes it possible to clean up oil spills, protect water sources from contamination, and use oil in various industries. It is clear that understanding why oil and water don’t mix is essential to our everyday lives.
