Water softening is a process that has been used for centuries to remove minerals from hard water. It is a process that has gained more popularity in modern times due to the fact that it improves the quality of water for several different purposes. The chemistry behind water softening is complex, but understanding how it works can help you to better understand why it is so important.
In this article, we will explore the science behind water softening and how the chemistry works to remove minerals from hard water. We will look at how the process works, the benefits of water softening, and the potential drawbacks of using a water softener system. By the end of this article, you will have a better understanding of the chemistry behind water softening and how it can help improve the quality of your water.
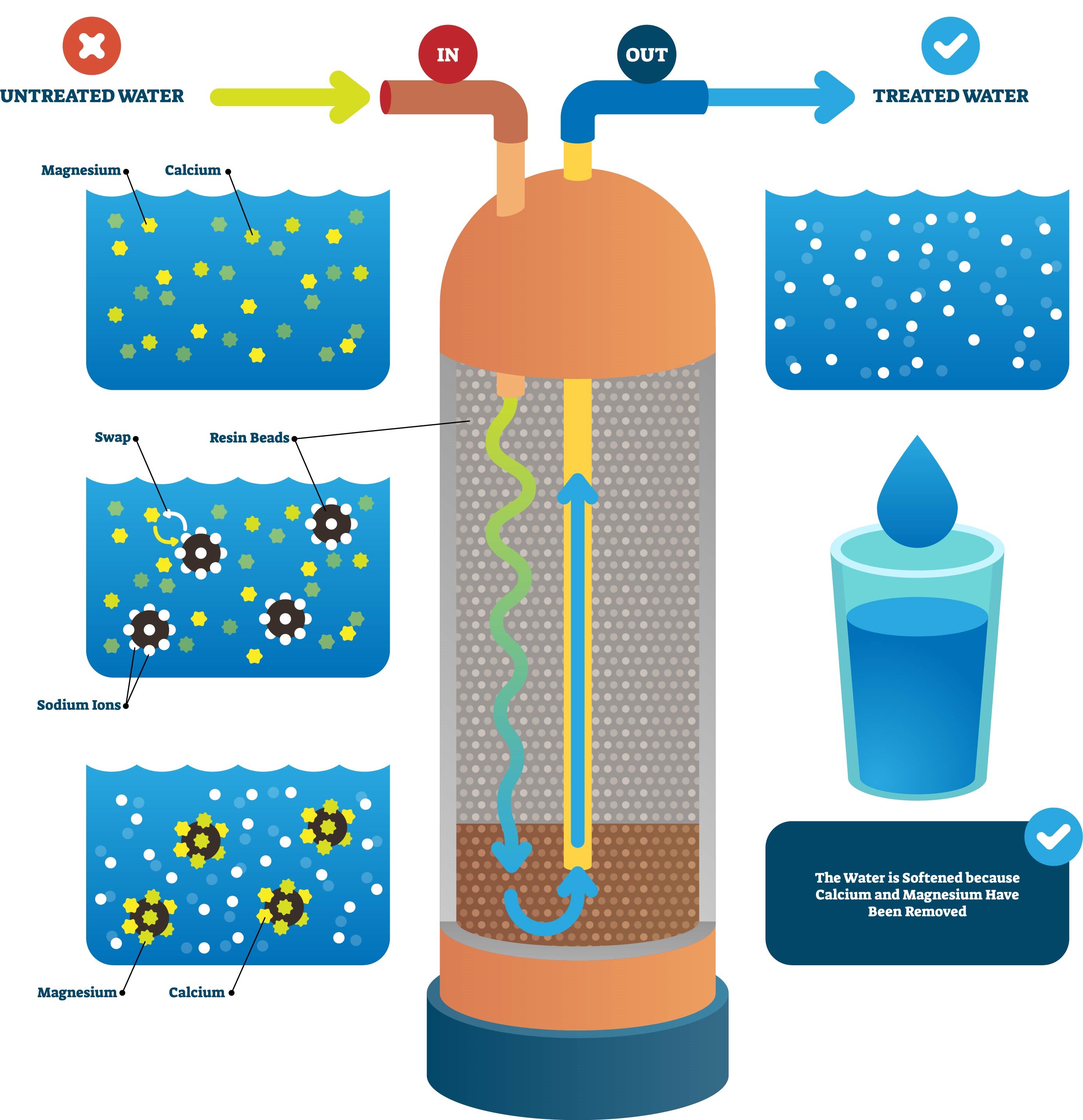
Water softeners work by removing hardness minerals from water using a process called ion exchange. This process involves exchanging sodium ions in the softener resin with calcium and magnesium ions found in the water. The sodium ions replace the calcium and magnesium, resulting in soft water.
Ion exchange water softening units are most commonly used in homes. They work by passing hard water through a bed of resin beads that contain an exchangeable sodium ion. As hard water passes through the resin beads, the calcium and magnesium ions are exchanged for sodium ions.
Once the resin bed is saturated with hardness ions, it must be regenerated. This is done by flushing it with a brine solution. The brine solution contains high levels of sodium and chloride ions, which are used to release the calcium and magnesium ions and replenish the exchangeable sodium ions.

How Does Water Softener Work Chemistry?
Water softeners are a common household appliance that use a process called ion exchange to reduce the levels of hard minerals such as calcium and magnesium in hard water. This process helps to prevent the buildup of limescale and makes water more suitable for drinking and cleaning. The chemistry of water softening involves several complex chemical processes, but in a nutshell, the process works by exchanging hard ions such as calcium and magnesium with sodium ions, which are less likely to form scale.
Ion Exchange
The process of ion exchange involves the exchange of ions between two solutions. In a water softener, the exchange takes place between hard water and a brine solution. The brine solution contains sodium chloride, which is also known as common table salt. As the hard water passes through the water softener, the calcium and magnesium ions in the water are exchanged with sodium ions from the brine solution. This process helps to reduce the levels of hard minerals in the water, making it softer.
The process of ion exchange is reversible and the sodium ions will eventually be replaced with calcium and magnesium ions. To prevent this from happening, the water softener uses a regeneration cycle. During the regeneration cycle, a concentrated brine solution is used to flush out the calcium and magnesium ions and replace them with sodium ions. The regeneration cycle helps to ensure that the water softener is able to continue to produce soft water.
Filtration
Water softeners also use a process called filtration to remove particulates from the water. A filter cartridge is used to trap suspended particles such as dirt and rust. This helps to further improve the quality of the water and prevents the buildup of scale in water-using appliances.
Filtration also helps to reduce the amount of chlorine in the water. Chlorine is added to water by municipal water treatment facilities to kill bacteria, but it can also cause skin irritation and an unpleasant odor. The filter cartridge helps to reduce the amount of chlorine in the water, making it safer to drink and use for other purposes.
Water Softener Resin
At the heart of a water softener is a special type of resin material. The resin is a bead-like material that is made from a high-grade plastic. The beads are coated with sodium ions and when the hard water passes through the resin, the calcium and magnesium ions in the water are attracted to the sodium ions on the resin. This helps to reduce the levels of hard minerals in the water.
The resin also needs to be regenerated regularly to ensure that it continues to work effectively. During the regeneration cycle, a concentrated brine solution is used to flush out the calcium and magnesium ions and replace them with sodium ions. This helps to ensure that the resin is able to continue to provide the soft water.
Salt
Salt is used in the regeneration cycle to help flush out the calcium and magnesium ions and replace them with sodium ions. The salt also helps to prevent the buildup of scale in water-using appliances. The amount of salt used in a water softener varies depending on the type of water softener and the hardness of the water.
Salt is also used to replenish the sodium ions on the resin beads in the water softener. The salt is added to the brine solution in the regeneration cycle and is used to replace the calcium and magnesium ions that have been removed from the water. This helps to ensure that the water softener is able to continue to produce soft water.
Frequently Asked Questions: How Does Water Softener Work Chemistry?
Water softeners are devices that are used to remove charged ions from hard water, such as calcium and magnesium, to make it softer and more suitable for use in everyday applications. The chemistry behind water softeners is complex but essential to understand so that you can make the most of your softener and improve your water quality.
What Are the Benefits of Softened Water?
Softened water can have a number of benefits, both practical and aesthetic. It can help reduce the amount of soap, detergent and shampoo used in the home, as well as reducing the amount of scale buildup in pipes and appliances. Softened water also makes it easier to achieve a good lather when showering or washing dishes and laundry, as well as providing a more pleasant taste and smell when used for drinking and cooking.
How Does a Water Softener Work?
A water softener works by passing hard water through a tank that contains a bed of resin beads. The resin beads are charged with sodium ions, which attract and bind the calcium and magnesium ions in the hard water. The softened water is then passed out of the tank and into the home, where it can be used for drinking and washing.
What Are the Components of a Water Softener?
The main components of a water softener are the resin tank, the resin beads, the brine tank and the control valve. The resin tank holds the resin beads, which are charged with sodium ions to attract and bind the calcium and magnesium ions in the hard water. The brine tank is filled with salt, which is used to regenerate the resin beads on a periodic basis. The control valve regulates the operation of the softener, controlling the amount of water that is allowed to pass through the tank and the frequency of regeneration cycles.
Why Do Water Softeners Need to Be Regenerated?
Water softeners need to be regenerated on a regular basis to maintain their effectiveness. As the resin beads bind to the calcium and magnesium ions in the hard water, they eventually become saturated and can no longer effectively remove the ions. To restore the softener’s effectiveness, the control valve will initiate a regeneration cycle, in which the brine tank flushes salt water through the resin tank to release the calcium and magnesium ions and restore the charge of the resin beads. This process must be repeated regularly in order to maintain the effectiveness of the softener.
What Are the Different Types of Water Softeners?
There are two main types of water softeners: ion exchange water softeners and salt-free water softeners. Ion exchange water softeners are the most common type and work by passing hard water through a tank containing resin beads charged with sodium ions. Salt-free water softeners work by passing hard water through a tank containing a filtration media which alters the chemical composition of the water, making it softer without adding any salt.
How do Water Softeners Work Chemistry
Water softeners are an integral part of modern households that rely on hard water. Understanding how they work is important for anyone who wants to maintain an efficient water system. Water softening works by using an ion exchange process to remove the hard minerals found in hard water. The exchange process removes calcium and magnesium ions and replaces them with sodium ions. This process results in softened water that is free from hard minerals and is much more suitable for everyday use.
The chemistry behind water softening is complex, but the results are undeniable. Water softeners are essential for households and businesses that rely on hard water. Not only does softened water reduce limescale build-up, it also helps extend the life of pipes and appliances. With a basic understanding of the process, anyone can use a water softener with ease to enjoy the benefits of softened water.
